✈️ Next Day Delivery Available
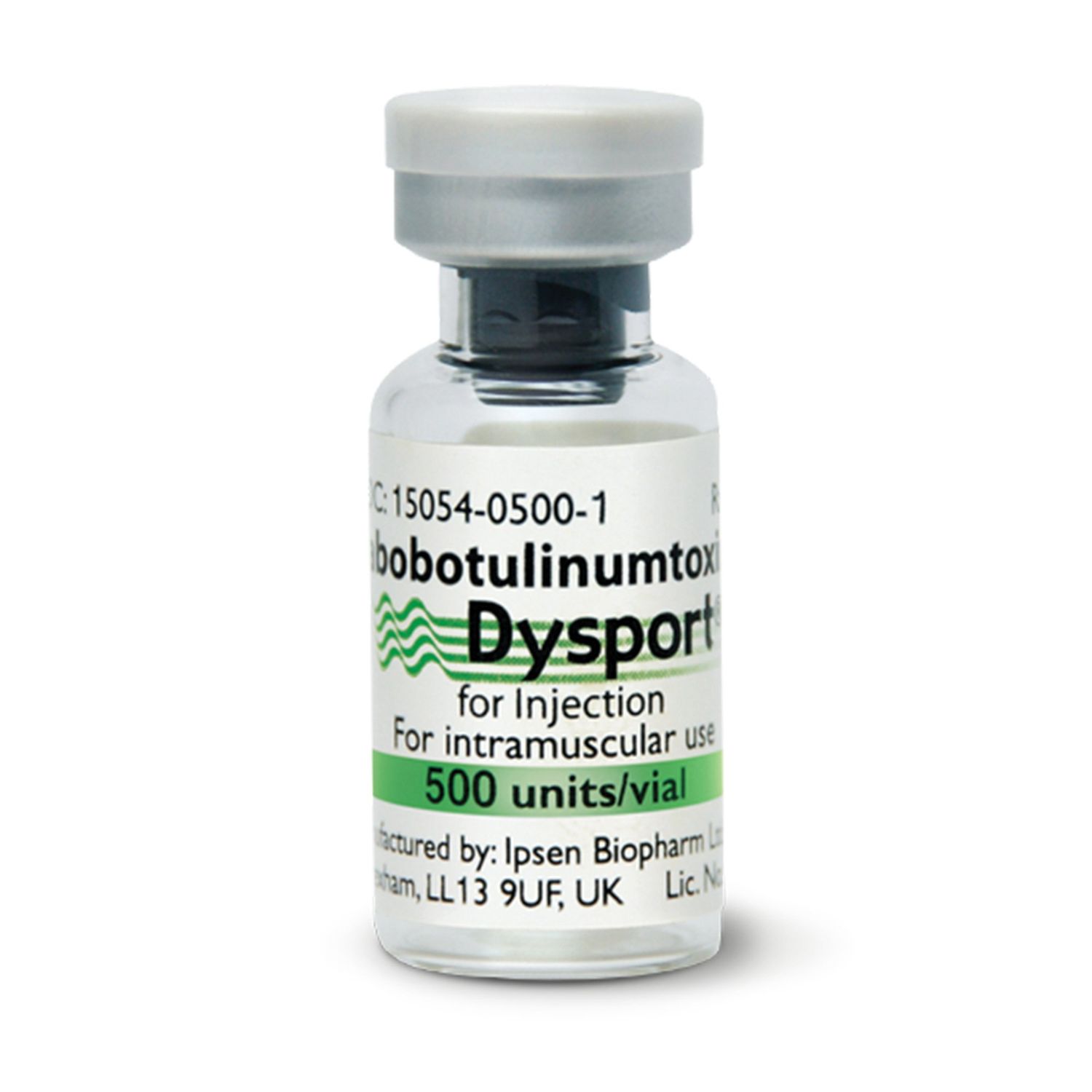
Dysport 500 units/vial
$289.00
4 payments of $72.25 with
In stock: 9 available
Save this product for later
Dysport 500 units/vial
Product Details
Dysport® (abobotulinumtoxinA) – 500-Unit Vial
Prescription-only botulinum toxin type A for intramuscular injection
Description
• Sterile, lyophilized powder for reconstitution.
• Each single-use vial contains 500 units of abobotulinumtoxinA, human serum albumin, and lactose.
• Once reconstituted with preservative-free 0.9 % sodium chloride injection, the solution is clear and colorless.
Indications (U.S. FDA–approved)
• Treatment of moderate to severe glabellar lines (adults).
• Cervical dystonia to reduce severity of abnormal head position and neck pain (adults).
• Upper limb spasticity (adults and children ≥2 yrs).
(Indications vary by country; confirm local regulatory labeling.)
Dosage & Administration
• Reconstitute with preservative-free 0.9 % NaCl to desired concentration (typical: 1.25 mL for 500 U).
• Use within 24 hours of reconstitution; store refrigerated 2–8 °C.
• Administer via intramuscular injection only, by qualified healthcare professionals.
Storage & Handling
• Store vials unopened at 2–8 °C (36–46 °F). Do not freeze.
• Protect from light.
• Single-use vial—discard any unused reconstituted solution.
Packaging
• Carton contains one (1) 500-Unit single-use vial with flip-off cap and tamper-evident seal.
• NDC (U.S.): 15054-0500-xx (check local market code if outside U.S.).
Key Safety Information
• Boxed Warning: May spread from injection site to produce botulinum toxin effects (e.g., dysphagia, respiratory compromise).
• Contraindications: Infection at injection site, known hypersensitivity to botulinum toxin or formulation components.
• Common adverse reactions: Injection-site pain, headache, nasopharyngitis, muscle weakness (varies by indication).
• Use only under supervision of licensed healthcare professionals.